Analysis of Ion Concentration Changes in Transverse Axial Tubular System in a Model of Human Ventricular Cardiomyocyte
Dana Ohlídalová, Michal Pásek*, Jiří Šimurda
Department of Physiology, Faculty of
Medicine,
*pasek.avcr@centrum.cz
The
cardiac transverse-axial tubular
system (TATS) is a complex network of membrane invaginations. Both structural
and functional studies have suggested that the TATS constitutes a region that
is highly specialised for excitation-contraction coupling because many of the
key proteins involved in transmembrane Ca2+ flux appear to be
located predominantly in the TATS (Orchard et al., 2009). In this paper, we
present a model of human ventricular cardiomyocyte incorporating TATS and the
results of quantitative analysis of ion concentration changes in tubular lumen at
different stimulation rates.
A model originally proposed to explore the TATS properties in guinea-pig ventricular myocyte (Pásek et al., 2008) was adopted and modified to meet the recently published results of electrophysiological experiments on isolated human cardiomyocytes (e. g.Gui-Rong et al. 1998).
|
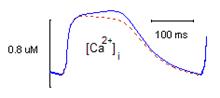
Fig. 1. Steady state intracellular Ca2+-transients
([Ca2+]i) under presence
(red dotted line) and absence (solid blue
line) of ion concentration changes in TATS during 3 Hz stimulation. |
Exploration of activity-dependent changes in tubular ion concentrations revealed a relative Ca2+ depletions of about 8 % in the range of stimulation frequencies 1-3 Hz. Meanwile, as the stimulation rate increased the relative maximum accumulation of tubular K+ decreased from 1.7 % to 0.7 %. The relative changes of tubular Na+ were negligible. The preliminary analysis of the effects of tubular Ca2+ depletion indicates its role in limitation of intracellular Ca2+ overload (Fig. 1). The detailed exploration of physiological consequences of this phenomenon is the subject of our further investigation.
References
Orchard CH, Pásek M, Brette
F. The role of
mammalian cardiac t-tubules in excitation contraction coupling: experimental
and computational approaches. Exp
Physiol, 94:
509 – 519, 2009
Pásek M,Šimurda J, Orchard C, Christé G. A model of the guinea-pig ventricular cardiac myocyte incorporating a transverse-axial tubular system. Progress Biophys and Mol Biol, 96: 258 – 280, 2008
Gui-Rong L, Feng J, Yue L, Carrier M. Transmural heterogenity of action potentials and Ito1 in myocytes isolated from the human right ventricle. Am J Physiol Heart Circ Physiol, 275: 369 – 377, 1998